The correct answer is A
The answer is C. The salt is not bound together and is all over the container.
The answer is B. There is no bound salt in this test tube but there is in the other test tubes.
The answer is B
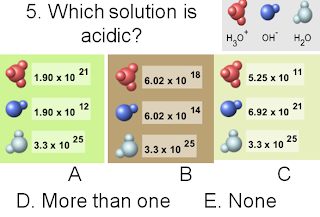
The answer is A
Teaching Idea-Density for First Grade
Monday: In the beginning of the week I will discuss with my class density beginning with density counting activity. We will have two large clear containers filled with water and we will use legos or another toy that the children are interested in. We will count equal amounts of legos inot each container counting as a class. I will then have a student remove a certain amount of legos and add them to the other container. Then I will ask the students which container has more density and why. The point to get across is that even though they take up the same amount of space one bucket has more.
Tuesday: Suitcase Activity. I will have two equal size suitcases and split the class into two groups. We will then use clothes from the dramatic play area and other miscellaneous items and I will time each group to see how much they can get into the suitcases. Then we will see which suitcase has more density.
Wednesday: I will give children different size balls, and we will measure their circumference. Then we will make a graph and I will have children graph and make a hypothesis about which ball they think will have the most density. Then we will do our experiment and see if their hypothesis is correct.
Thursday: On Thursday (or whatever day we have computer lab) I will have the children do the mystery lab on the density simulation and answer the worksheet that I provide. The children will look at the simulation before hand and make guesses about which blocks will sink or float. Then the children will complete the simulation and answer if their hypthesis were correct or not. They can then play with the rest of the simulation.
Friday: On friday the children will be asked to bring in an item from home that they think will out sink or out float thier classmates. We will then make hypothesis about what we think about the students items. We will then do the experiment to see if after everything they have learned during the week if they can positively decide what will sink and what will float.
Standards:
C.4.2 Use the science content being learned to ask questions, plan investigations, make observations, make predictions, and offer explanations.
A.4.1 When conducting science investigations, ask and asnwer questions that will help and decide the general areas of science being addressed.
C.4.5 Use data they have colelcted to develop explanantions and answer questions generated by investigations.